Can I prescribe naloxone to a caregiver not at risk for overdose?
Who should be educated in the use of a naloxone rescue kit? How should they be identified?
The highest risk patients are people who resume use after a period of abstinence with reduced opioid tolerance, such as people released from incarceration or leaving substance use disorder treatment.
Who should provide the education? How long does it take?
How should providers/staff be trained?
What about liabilities and risk following injection or naloxone administration errors?
What are the results of other programs like this/who else is doing this work?
The promise of naloxone rescue kits has been recognized through endorsements by the United Nations Office on Drugs and Crime (UNODC) jointly with the World Health Organization (WHO), US President’s Emergency Plan For AIDS Relief (PEPFAR), the American Public Health Association (APHA), the American Medical Association (AMA), the Office of National Control Policy (ONDCP), state legislatures, public health departments and national programs.
Why is prescribing naloxone superior to basic life support and calling 911?
The current AHA guidelines describing “Hands-Only CPR” have received widespread publicity in the media, without mention of the accompanying exceptions for situations involving drug overdose, drowning, or collapse due to breathing problems. People who have received CPR training recently may have been encouraged to discontinue rescue breathing universally, as opposed to the more appropriate context of during sudden collapse or a heart attack.
How much does the naloxone cost? Who is paying for the naloxone?
Most insurance, Medicaid and Medicare will pay for naloxone, but coverage varies by state. The nasal adapter is not covered by insurance. Compiling a list of insurance plans in your area that will cover naloxone and collaborating with a pharmacist will be helpful. Because naloxone is not an expensive medication, even patients whose insurance will not cover the medicine may be willing to pay for naloxone. Advocates in some locations have been successful in persuading insurers to cover the cost of naloxone or have it added to local ADAP (AIDS Drug Assistance Program) formularies.
How can I bill for the training time?
What is the liability of prescribing naloxone? What if they use it on someone else (medication is not prescribed to them/ use is not as directed)?
As with any prescription medication, patients receiving a naloxone prescription should receive verbal and written instructions on how and when to use this drug. Prescribers/staff should not instruct patients to administer naloxone to persons who do not have a valid prescription for the drug; however, both prescribers and patients can educate a potential bystander how to administer the medication to the patient during an overdose emergency.
Are there adverse events associated with naloxone administration?
How do I decide on a naloxone formulation?
0.4/mL naloxone for intramuscular (IM) injection only is available in two forms from Hospira (NDC 0409-1219-01 for 10mL multidose vial and NDC 00409-1215-01 for 1mL single dose vial)
Advantages:
• First line for paramedics & emergency departments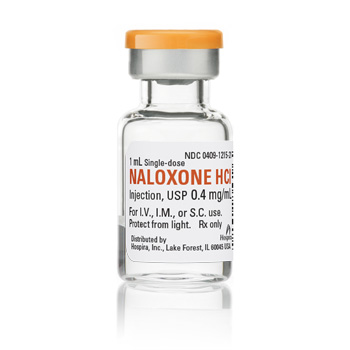
• Most commonly used formulation
• Comparatively intuitive assembly and administration
• Manufacturer more accessible for strategizing, negotiating, planning
• FDA approved (IMS/Amphastar product is also FDA approved, but not for IN administration & we discourage IM/IV administration of that stronger formulation by laypersons because it requires dosing decisions)
Disadvantages:
• Needle use seems to cause generic anxiety in many groups (though people are consistently able to effectively use the needles in emergencies)
• Needle disposal requirements
2mg/2mL naloxone is available from IMS/Amphastar (NDC 76329-3369-1) is modifiable post market to be useable intranasally (IN)
Advantages:
• First line for some local EMS 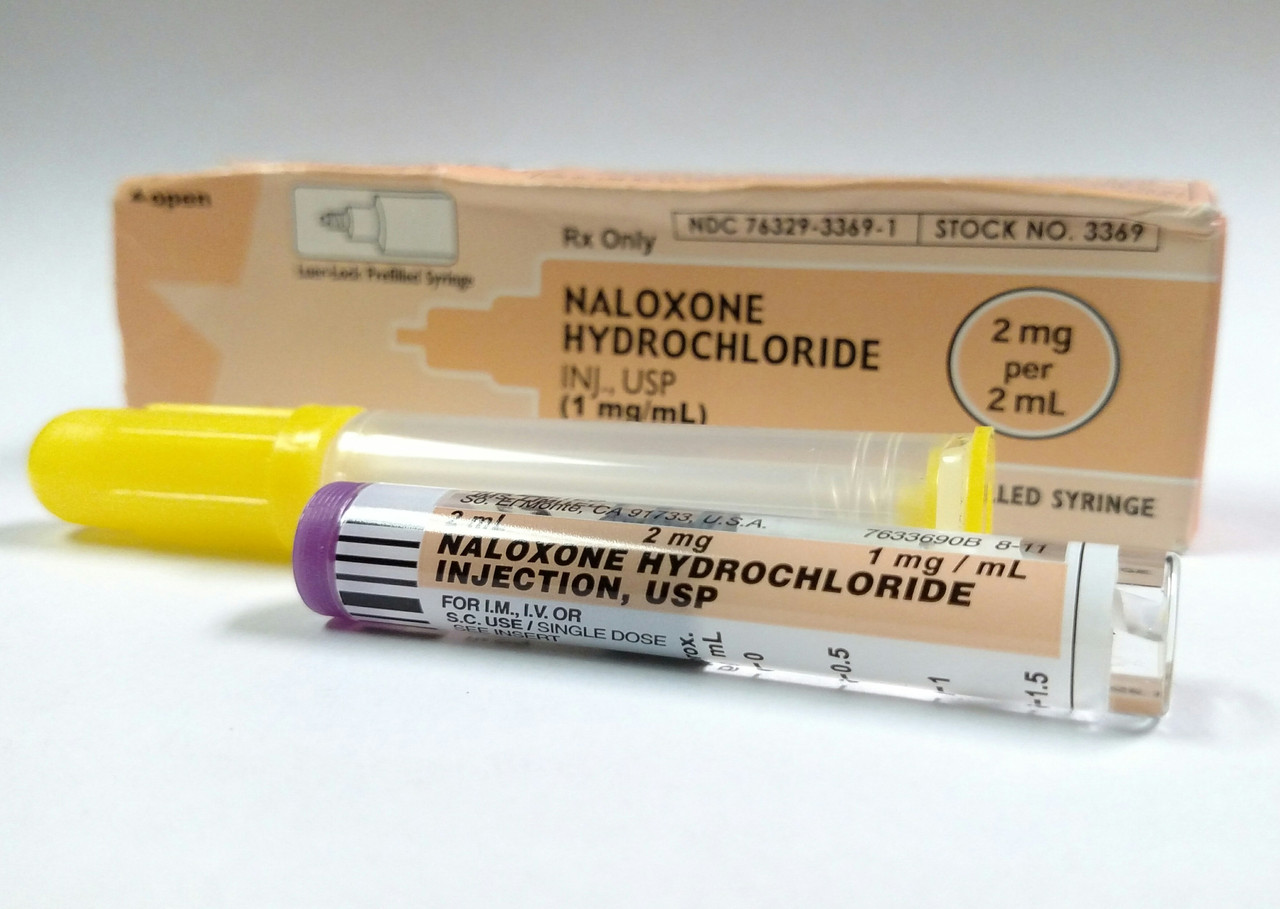
• Randomized Control Trials (RCT): slower onset of action but milder withdrawal
• Acceptable to needle-adverse groups
• No needle stick risk
• No needle disposal concerns
Disadvantages:
• Not FDA approved
• No large RCT
• Assembly required, not intuitive
• Subject to breakage
• Cumbersome commercial pharmacy sourcing for MAD 300 nasal adapter from Teleflex
Products approved by FDA in 2014– PrescribeToPrevent.org does not have sufficient experience with either product to comment on advantages and disadvantages:
0.4/mL naloxone generic from Mylan (NDC 67457-292-02 for 1mL single dose vial)- no image available
0.4/mL naloxone auto-injector called Evzio® is available from kaléo (NDC 60842-030-01 for 2 auto-injectors + 1 trainer)
Should I be concerned about naloxone storage?
Extreme and repeated temperature fluctuations have been shown in a simulation study to degrade the concentration of the medicine over time. The study by Gammon and colleagues was done in the context of existing EMS, so this issue is one that is unresolved in the EMS context as well as in the expanded context of police, fire or laypersons. The degradation weakens concentration, but has not shown toxic byproducts, etc. This means that a person may, in the scenario with extreme temperature changes, receive a lower dose, but would still be receiving naloxone. Layperson naloxone administration is proposed in addition to current status quo, not instead of it. As such, even in the worst case scenario, an overdose victim would receive a slightly weaker dose earlier on than they would have otherwise, thus moving the continuum of care more forward. Ultimately, even if the medicine were less concentrated than at the time of manufacture due to improper storage (which is a ubiquitous concern), that scenario is still likely more of a benefit than not having an equipped first responder of layperson present to administer the medicine.
For the context of deploying naloxone among police and fire first responders, we have compiled a summary of practices among existing pilots and recommendations.
Which naloxone product should we use?
Which is the correct resuscitation technique to teach lay overdose responders?
Which is the correct order of actions when responding to an overdose?